The carbon atom in graphene is sp2 Hybridised, having three bonds related to different neighbor carbon atoms. These three bonds are on the same sheet, and the angles between them are equal to 120 degrees. In this case, the carbon atoms create a lattice of regular hexagonal structure. The distance of the C-C bond in graphene is about 0.142 nm.
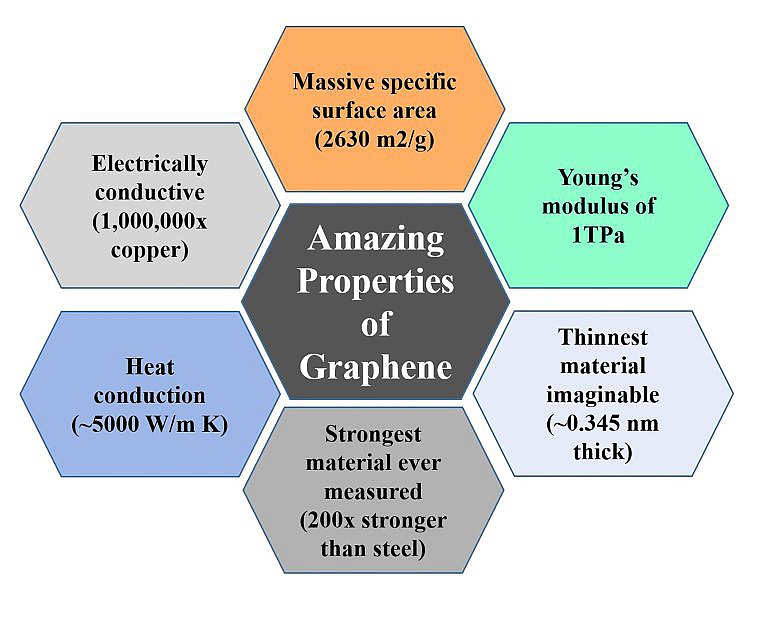
Graphene has received attention in sensors, bio-medicals, composite materials and microelectronics. Extensive applications such as transparent conductive films, ultra-sensitive chemical sensors, thin-film transistors, quantum dot devices, and anti-corrosion coverings have been tested and proven effective. However, industrial-scale graphene production for these overwhelming applications highly depends on the simple graphene production method, which has been improved recently. The hexagonal 2D crystalline single layer of carbon atoms that contains various Oxygenated functional groups such as epoxy, carboxyl, and hydroxyl is called graphene oxide.